New Market Opportunities
Combining ECG with PPG opens new markets and defends against competitive threats. Three main opportunities are unlocked when clinical-grade ECG is provided, and they all start with physician support:
- Physician recommendations to patients and loved ones.
- Open the opportunity to the B-to-B healthcare market with Payers, Clinical Research Organisations, and Employers.
- Gain access to reimbursed programs such as Remote Patient Monitoring, Transition Care Monitoring, Chronic Care Monitoring.
And unlike step counts or sleep scores, ECG is harder to commoditise. It requires regulatory oversight and higher validation efforts compared to simpler PPG-based features, raising the bar for accuracy and trust. This makes ECG a differentiator that adds lasting value, rather than a feature that can be easily copied or mass-produced.
What’s Holding You Back?
Companies might hesitate about adding ECG due to perceived barriers. Let’s break them down.
“Regulation is too complex.” Adding ECG for heart rhythm analysis brings regulatory requirements, such as ISO 13485 compliance and securing a 510(k) or CE Mark to sell your product in the US or European markets. However, partners like B-Secur can help you efficiently navigate this process. We have achieved two FDA 510(k) clearances on our own device agnostic ECG software products (K200884 and K233755) and have 5+ years of expertise helping consumer brands add ECG into their wearables.
“It’s too expensive or adds complexity.” ECG hardware design used to require multiple electronic components and extensive engineering. Today, highly integrated Analog Front Ends (AFEs), electrode evaluation kits and off-the-shelf algorithm solutions dramatically reduce time to market and cost. B-Secur offers products and professional services to help you achieve your wearable goals.
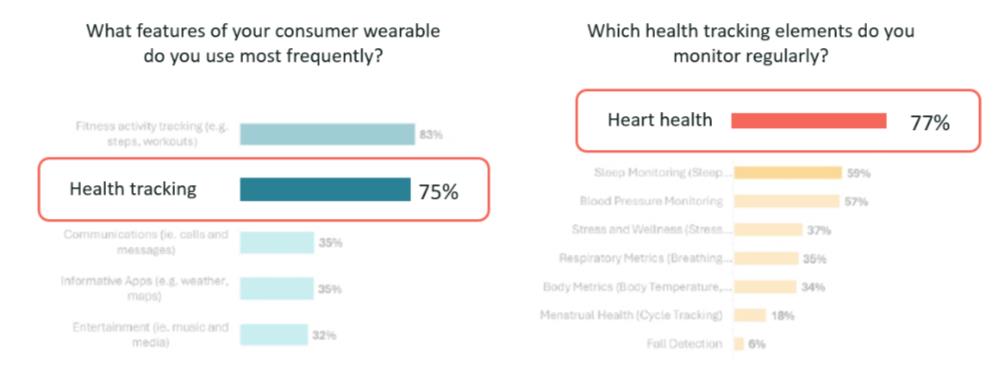
“Users don’t care.”. On the contrary—there is strong and growing consumer interest in heart health tracking. Our recent survey showed that users increasingly seek wearables not just for fitness, but for early warning signs and health and wellness management. The rise of ECG-enabled smartwatches from major brands has helped educate consumers about conditions like Atrial Fibrillation, while increased media coverage and awareness of heart health risks—especially in ageing populations—has fuelled demand.
The question isn’t whether to add ECG— It’s how quickly you can integrate it in a way that sets you apart.
At B-Secur, we help wearable companies integrate ECG with confidence regardless of the form factor and specific needs—from ECG signal chain design, to signal quality evaluation, pre-compliance testing, regulatory support, and 510(k) cleared ECG algorithms.
Whether you’re exploring feasibility or preparing a product launch, we can help shorten your path to market.
1. https://newsnetwork.mayoclinic.org/discussion/ai-transforms-smartwatch-ecg-signals-into-a-diagnostic-tool-for-heart-failure/
2. https://www.heart.org/en/news/2024/06/04/heart-disease-and-stroke-could-affect-at-least-60-percent-of-adults-in-us-by-2050
3. https://www.health.harvard.edu/mens-health/three-times-as-many-people-have-atrial-fibrillation-than-previously-known
4. https://newsroom.heart.org/news/more-than-half-of-u-s-adults-dont-know-heart-disease-is-leading-cause-of-death-despite-100-year-reign
5. Wearable Healthcare Devices Market worth $69.2 billion by 2028 https://www.marketsandmarkets.com/PressReleases/wearable-medical-device.asp?utm_source=chatgpt.com
6. Wearable Medical Devices Market Trends https://www.grandviewresearch.com/industry-analysis/wearable-medical-devices-market
7. Hemingway, J. et al. (2021). Photoplethysmography for the detection of atrial fibrillation: A systematic review and meta-analysis.* Australasian Physical & Engineering Sciences in Medicine, 44, 613–633. https://doi.org/10.1007/s13246-021-01072-5
8. https://afiponline.org/articles/weighing-the-effectiveness-of-photoplethysmography-signal-analysis-in-population-diagnosis-of-atrial-fibrillation
9. De Bie et al., 2021
10. https://www.frontiersin.org/journals/cardiovascular-medicine/articles/10.3389/fcvm.2022.869730/full
11. https://www.apple.com/uk/healthcare/docs/site/Apple_Watch_Arrhythmia_Detection.pdf
12. https://www.frontiersin.org/journals/cardiovascular-medicine/articles/10.3389/fcvm.2022.869730/full